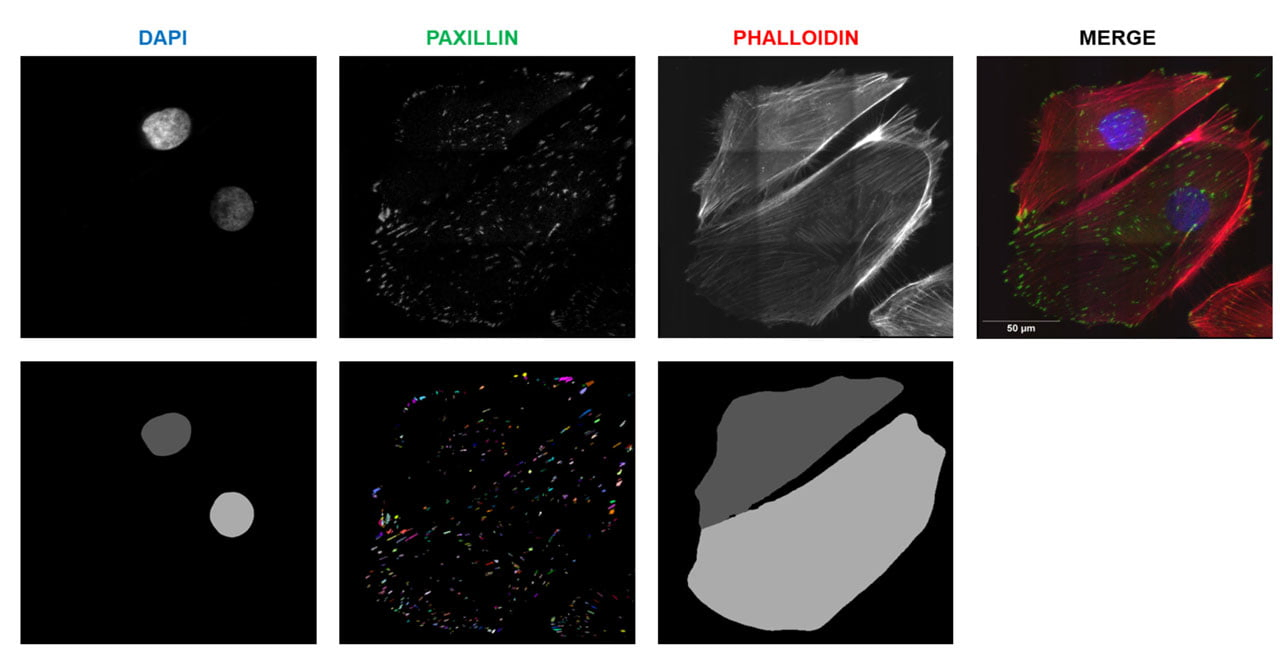
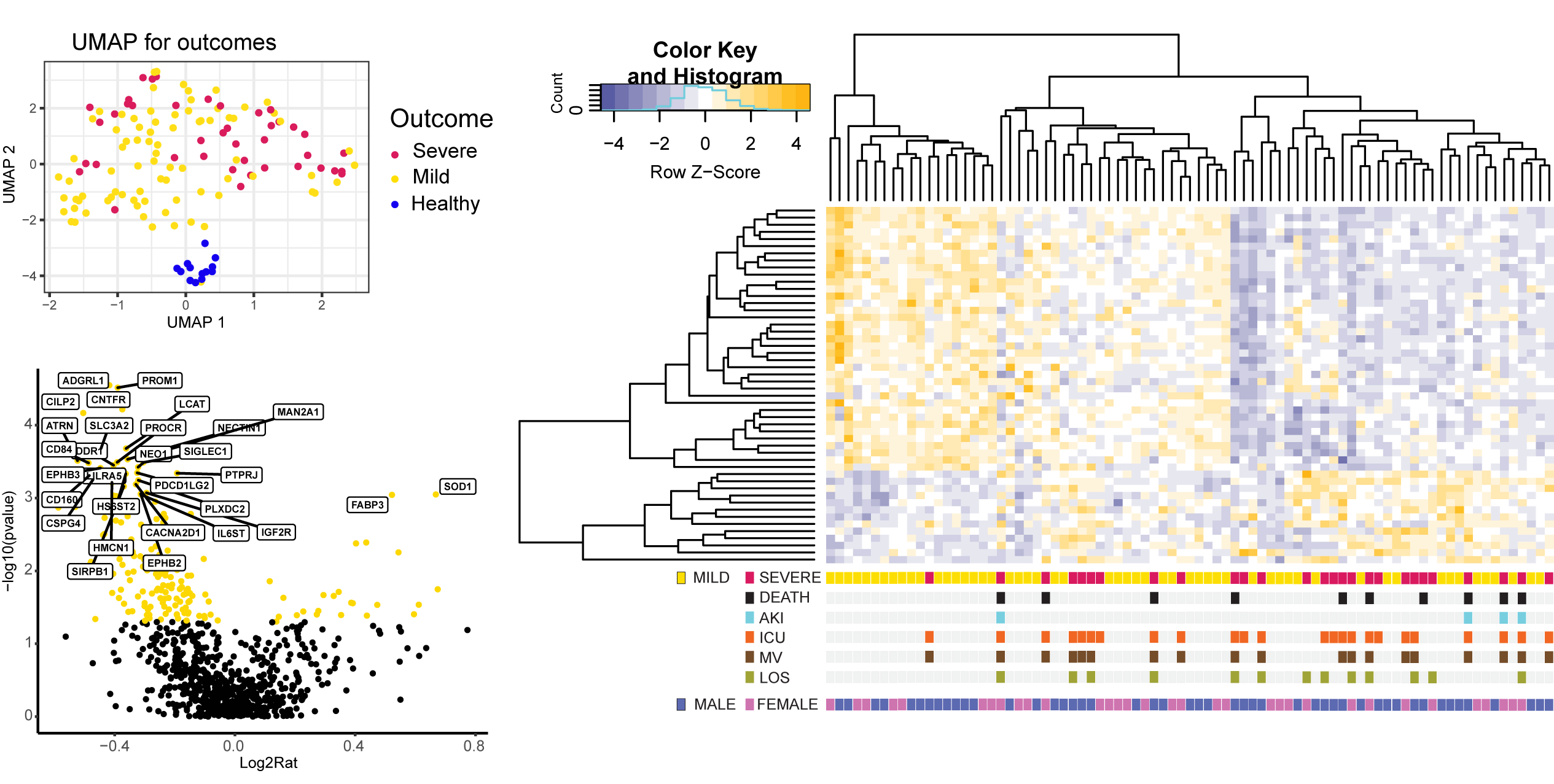
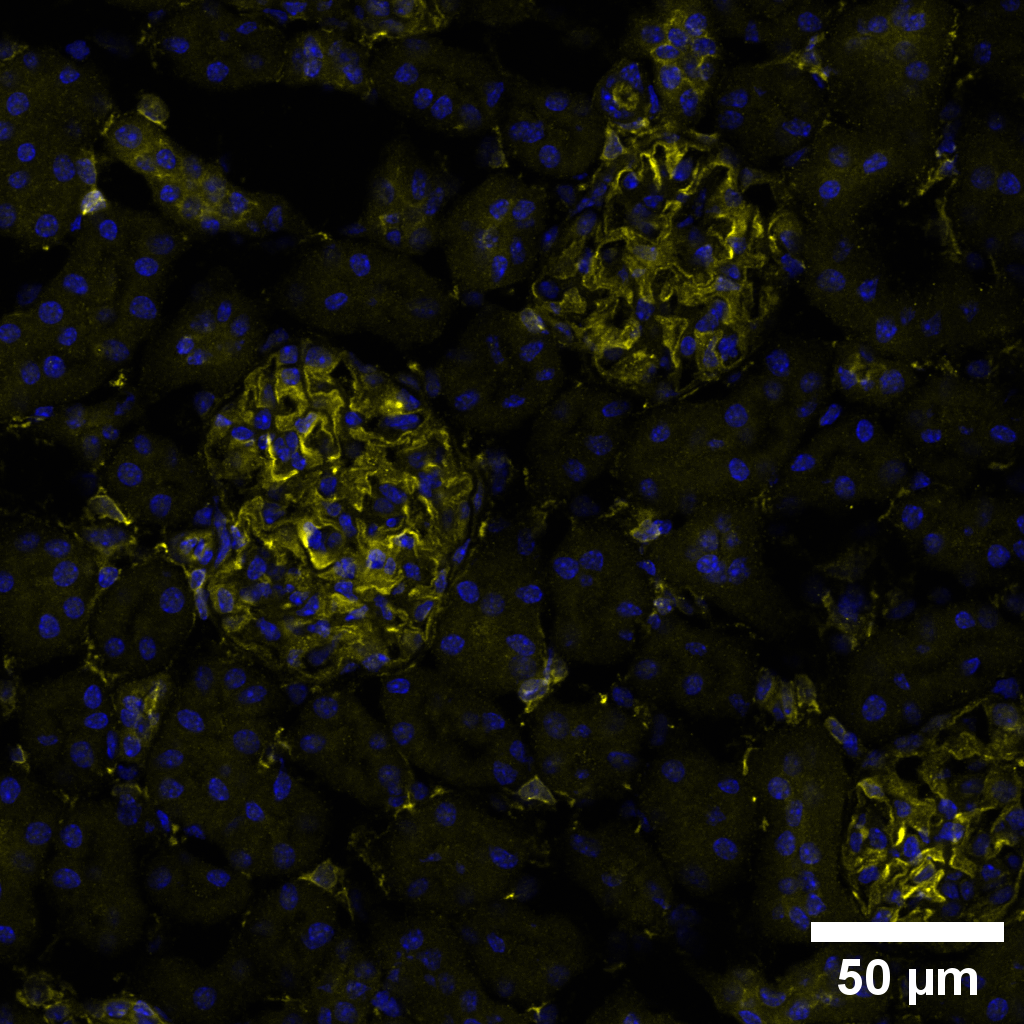
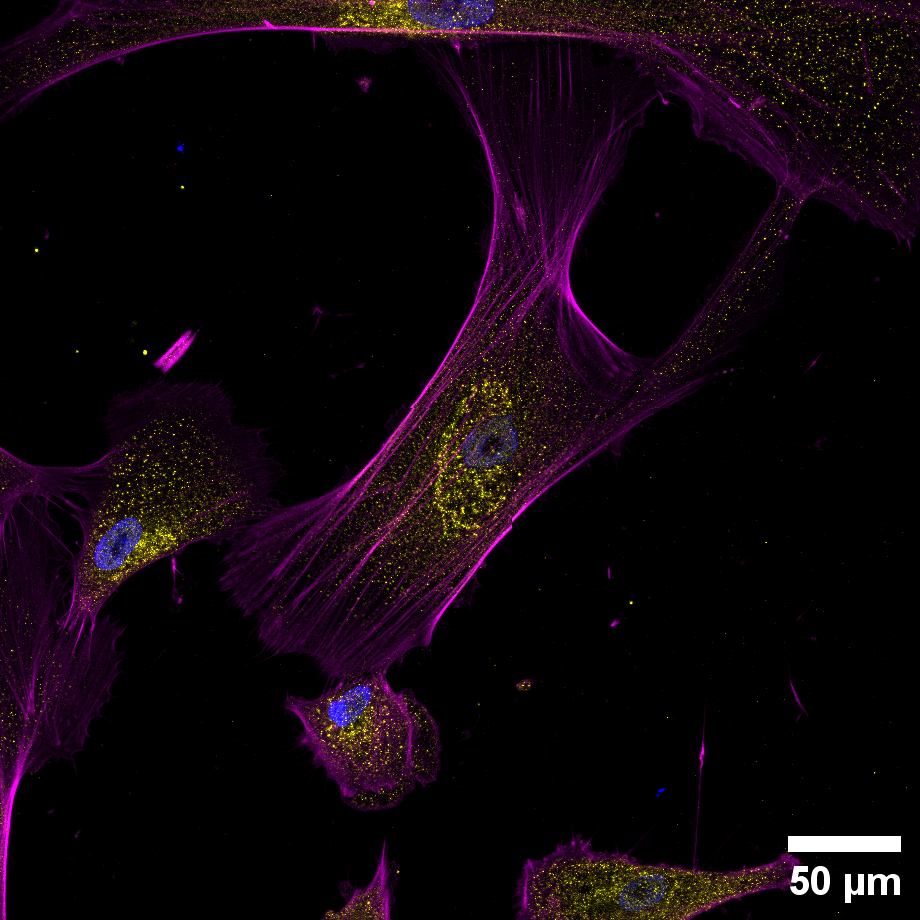
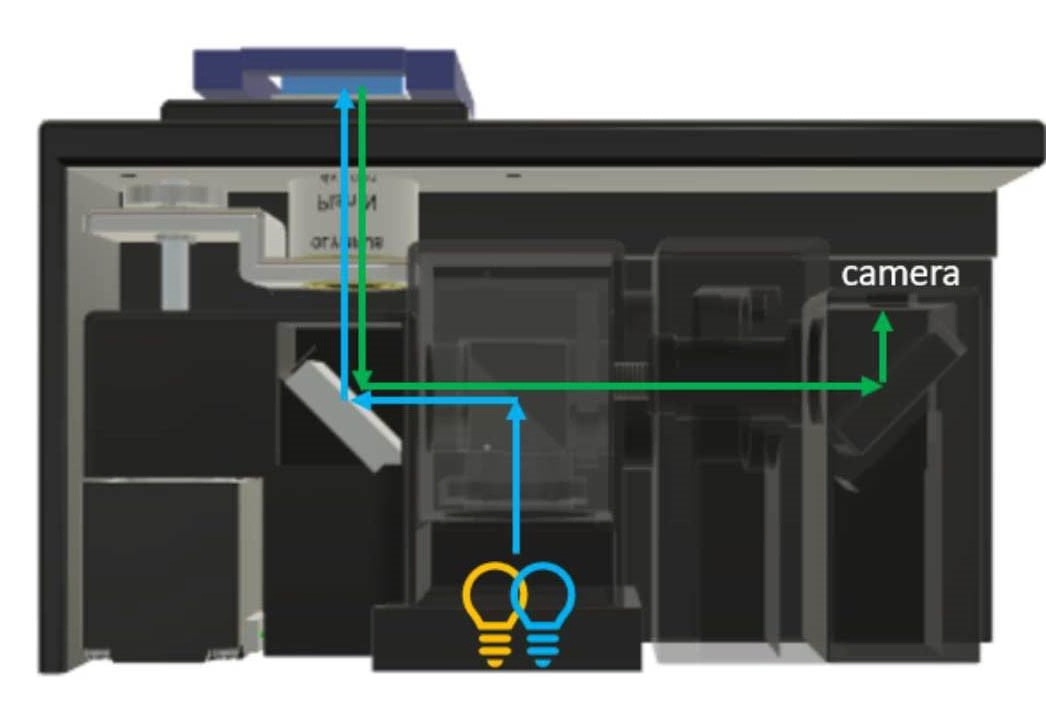
research
How the interaction of physical forces and biochemical processes affects basic cell biology still eludes most scientists. We study systems-level regulation of cell and tissue biomechanics to understand its role in progression of complex diseases, and to discover new drug targets and to design precision therapeutic strategies based on mechanobiological principles. We are specifically interested in proteins that form the focal adhesion complex and actin-associated proteins that shape the mechanobiological information processing capacity of the cell. In addition to playing critical structural roles, these crosslinking and adapter proteins modulate mechanotransduction through spatial segregation of signaling proteins. We use proteomics to identify key functional proteins within the cytoskeleton or the adhesome and utilize classical cell biological as well as bioengineering methods to characterize the complex role they play in pathophysiology. We have recently showed how multiple nested network motifs-controlled expression and localization of the actin-crosslinking protein, synaptopodin, in kidney podocytes. In the past our findings have been featured as the cover story on Science Signaling. And more recently, we published a Nature Reviews Nephrology article detailing the core biophysical and biomechanical principles associated with podocyte physiology and further discuss mechanobiological pathways that could be harnessed for the discovery of podocyte-specific therapeutics.